卵母细胞 COIP (Co-Immunoprecipitation in Oocytes) is a crucial technique in molecular biology used to study protein-protein interactions within oocytes, or egg cells. As reproductive biology advances, understanding the complex interplay of proteins in oocytes becomes essential for insights into fertility, embryogenesis, and developmental biology. This article provides a comprehensive exploration of 卵母细胞 COIP, detailing its methodology, applications, challenges, and significance in research.
Table of Contents
ToggleWhat is 卵母细胞 COIP?
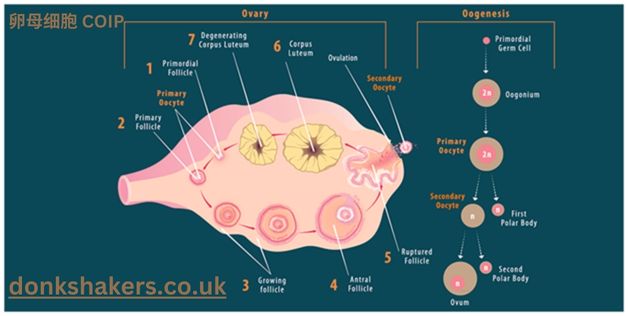
Definition of 卵母细胞
- 卵母细胞 (oocyte): A female gamete that undergoes maturation to form an ovum, playing a central role in reproduction.
- Oocytes contain unique protein networks essential for meiotic division, fertilization, and early embryonic development.
What is COIP?
- Co-Immunoprecipitation (COIP): A laboratory technique used to identify and analyze protein-protein interactions.
- Involves the use of specific antibodies to isolate a protein of interest and its interacting partners.
卵母细胞 COIP
Combines COIP with oocyte research to study critical protein interactions that influence oocyte development, maturation, and function.
Importance of 卵母细胞 COIP in Research
Understanding Protein Interactions
- Helps identify proteins involved in signaling pathways, structural support, and regulatory mechanisms in oocytes.
Applications in Fertility Studies
- Provides insights into the molecular causes of infertility and potential therapeutic targets.
Advancing Reproductive Technology
- Enhances techniques like in vitro fertilization (IVF) by understanding oocyte health and maturation.
Developmental Biology
- Unravels the role of maternal proteins during early embryogenesis.
key Steps in Performing 卵母细胞 COIP
1. Sample Preparation
- Isolate oocytes from the desired organism (e.g., mouse, human).
- Lyse the oocytes to release proteins while preserving their native interactions.
2. Antibody Selection
- Choose an antibody specific to the protein of interest.
- Ensure high specificity and minimal cross-reactivity.
3. Immunoprecipitation
- Incubate the lysate with the antibody bound to beads (e.g., agarose or magnetic).
- Capture the target protein and its interacting partners.
4. Washing and Elution
- Remove non-specific proteins through a series of washes.
- Elute the protein complex for downstream analysis.
5. Analysis
- Use techniques like SDS-PAGE and mass spectrometry to identify the proteins in the complex.
Factors Affecting 卵母细胞 COIP
1. Antibody Quality
- High-affinity antibodies yield better results and reduce background noise.
2. Protein Abundance
- Low-abundance proteins may require enhanced sensitivity techniques.
3. Oocyte Quality
- Ensure healthy, mature oocytes for reliable results.
4. Optimization of Buffer Conditions
- Maintain appropriate pH, salt concentration, and detergent levels to preserve protein interactions.
Applications of 卵母细胞 COIP
1. Fertility Research
- Identifies protein networks critical for oocyte maturation and ovulation.
- Studies genetic mutations linked to infertility.
2. Cancer Research
- Explores the role of proteins in oocyte tumors like teratomas.
3. Drug Development
- Identifies molecular targets for drugs that enhance or inhibit fertility.
4. Epigenetics
- Investigates maternal proteins influencing gene expression in embryos.
Challenges in 卵母细胞 COIP
1. Limited Sample Availability
- Oocytes are often scarce, requiring careful sample handling and efficient protocols.
2. Protein Stability
- Protein-protein interactions are sensitive to experimental conditions.
3. Non-Specific Binding
- Background noise can complicate data interpretation.
4. Technical Expertise
- Requires skilled personnel for accurate execution and analysis.
Advances in 卵母细胞 COIP
1. High-Throughput Techniques
- Automation of COIP for large-scale protein interaction studies.
2. Enhanced Sensitivity
- Use of advanced antibodies and detection methods to capture low-abundance proteins.
3. Integration with Omics Technologies
- Combines COIP with proteomics and genomics for comprehensive molecular insights.
4. Live-Cell COIP
- Emerging techniques allowing real-time study of protein interactions in living oocytes.
Case Studies: Applications of 卵母细胞 COIP in Research
Case Study 1: Identifying Meiotic Regulators
- Used to isolate proteins involved in oocyte meiotic spindle formation, aiding in understanding chromosomal segregation errors.
Case Study 2: Unraveling Fertility Disorders
- Identified mutations in interacting proteins that disrupt oocyte maturation, providing potential therapeutic targets.
Case Study 3: Embryo Development
- Explored maternal proteins influencing zygote polarity and division, contributing to IVF success rates.
Tips for Successful 卵母细胞 COIP
1. Optimize Conditions
- Tailor buffer compositions and incubation times to specific protein interactions.
2. Use Fresh Samples
- Process oocytes immediately after isolation to preserve interactions.
3. Validate Results
- Confirm findings through complementary techniques like Western blotting.
4. Collaborate with Experts
- Partner with immunology and proteomics specialists for advanced analysis.
Future Directions in 卵母细胞 COIP
AI-Driven Data Analysis
- Integration of artificial intelligence for pattern recognition in protein interaction networks.
Microfluidics
- Use of microfluidic devices for efficient handling of limited oocyte samples.
Single-Oocyte Analysis
- Development of methods to study protein interactions in individual oocytes.
Clinical Translation
- Application of 卵母细胞 COIP findings to improve diagnostics and treatment for infertility.
Conclusion: The Role of 卵母细胞 COIP in Modern Science
卵母细胞 COIP is a cornerstone technique in reproductive biology, offering deep insights into protein interactions essential for oocyte function and fertility. Despite its challenges, advancements in technology and methodology are expanding its applications, from basic research to clinical innovations. As scientists continue to explore the molecular intricacies of oocytes, 卵母细胞 COIP will remain a vital tool, shaping the future of reproductive medicine and developmental biology.